Invited speakers
(Universitat Zurich, CH)
Stefano GIANNI
(University of Roma, IT)
George MAKHATADZE
(Rensselaer Polytechnic Institute, USA)
Anne S. ULRICH
(Universitat Karlsruhe, DE)
Daniel MÜLLER
(ETH Zurich, CH)
Philipp SELENKO
(FMP Berlin, DE)
Martin VABULAS
(Goethe University Frankfurt, DE)
Robert BEST
(Cambridge University, UK)
Jan HEYDA
(Helmholtz-Zentrum für Materialien und Energie GmbH, Berlin, DE)
Michele VENDRUSCOLO
(University of Cambridge, UK)
(Johns Hopkins University, Baltimore, USA)
Peter TOMPA
(Hungarian Academy of Sciences, Budapest, HU)
Valerie DAGGETT
(University of Washington, Seattle, USA)
Gunnar von HEIJNE
(Univeristy of Stockholm, SE)
Vladimir UVERSKY
(University of South Florida, Tampa, USA)
Jim WARWICKER
(Manchester Interdisciplinary Biocentre, UK)
Dietmar PASCHEK
(Universitat Rostock, DE)
Zygmunt S. DEREWENDA
(University of Virginia, USA)
Kvido STRISOVSKY
(IOCB AS CR, Prague, CZ)
Mikael OLIVEBERG
(University of Stockholm, SE)
Scientific Program
Friday, May 4, 2012
8:30 – 8:40
Invitation by Zdeněk Havlas, IOCB AS CR director
Session 1. Proteins in Membranes
Chairman Andreas Plueckthun
1.1 08:40 – 09:20 Daniel Mueller (ETH Zurich, CH)
Reversible un- and refolding of single transmembrane beta-barrel proteins into membrane bilayers
1.2 09:20 – 10:00
Gunnar Von Heijne (Univeristy of Stockholm, SE)
Energetics and dynamics of translocon-mediated membrane-protein insertion
1.3 10:00 – 10:40
Kvido Strisovsky (IOCB AS CR, Prague, CZ)
Proteolysis in lipid membrane: substrate specificity and mechanism of rhomboids
10:40 – 11:00 Coffee Break
1.4 11:00 – 11:40 Anne Ulrich (Universitat Karlsruhe, DE)
Charge zippers: a novel motif for folding and self-assembly of membrane proteins
1.5 11:40 – 12:20 Valerie Daggett (University of Washington, Seattle, USA)
Proteins in different environments: the effect of osmolytes, lipids, and neighboring proteins on protein unfolding
12:30 – 14:00 Lunch
Session 2. Proteins in osmolytes, denaturants and salts
Chairman Valerie Daggett
2.1 14:00 – 14:40 George Rose (Johns Hopkins University, Baltimore, USA)
Protein domains – a thermodynamic definition
2.2 14:40 – 15:20 George Makhatadze I (Rensselaer Polytechnic Institute, USA)
Protein folding and aggregation: linking computation and experiment
2.3 15:20 – 16:00 Jim Warwicker (Manchester Interdisciplinary Biocentre, UK)
Continuum electrostatics models for biology
16:00 – 16:20 Coffee Break
2.4 16:20 – 17:00 Vladimir Uversky (University of South Florida, Tampa, USA)
Intrinsically disordered proteins in salted water and thick soup
2.5 17:00 – 17:40 Stefano Gianni (University of Roma, IT)
Nearly identical, yet very different: addressing the folding of proteins with increasing degree of sequence identities but different structure and function
19:30 – 23:00 Poster Session
Saturday May 5th
Session 2. Proteins in osmolytes, denaturants and salts
Chairman Valerie Dagett
2.6 08:30 – 09:10 Dietmar Paschek (Universitat Rostock, DE)
Computer simulation of the folding/unfolding equilibrium of the Trp-Cage miniprotein: understanding the effects of pressure and denaturants.
2.7 09:10 – 09:50 Jan Heyda (Helmholtz-Zentrum für Materialien und Energie GmbH, Berlin, DE) Different denaturation pathways of Trp-Cage Minipeptide triggered by urea and guanidinium chloride
9:50 – 10:10 Coffee break
Session 3. Proteins in cells
Chairman Peter Tompa
3.1 10:10 – 10:50 Phil Selenko (FMP Berlin, DE)
Residue-resolved IDP dynamics in live mammalian cells
3.2 10:50 – 11:30 Mikael Oliveberg (University of Stockholm, SE)
In cell NMR of the ALS-associated protein SOD1
3.3 11:30 – 12:10
Michele Vendruscolo (University of Cambridge, UK)
Characterization of free energy landscapes of proteins using NMR spectroscopy
12:10 – 14:30 Lunch
Session 4. Proteins in crystals, complexes, and aggregates
Chairman George Rose
4.1 14:30 – 15:10
Martin Vabulas (Goethe University Frankfurt, DE)
Amyloid proteotoxicity
4.2 15:10 – 15:50 Peter Tompa (VrijeUniversiteit Brussel, BE)
Structure and function of intrinsically disordered chaperones
15:50 – 16:10 Coffee Break
4.3 16:10 – 16:50 Andreas Plueckthun (Universitat Zurich, CH)
Engineering specific and sensitive detectors from synthetic binding proteins
4.4 16:50 – 17:30 Zygmunt Derewenda (University of Virginia, USA)
Are protein crystals just convenient and useful artifacts
4.5 17:30 – 18:10 Robert Best (Cambridge University, UK)
Passive role of a chaperonin cavity in modulating folding kinetics and preventing protein misfolding
18:10 General discussion and concluding remarks
19:30 – 23:00 Dinner at a steamboat – Sightseeing from Vltava River
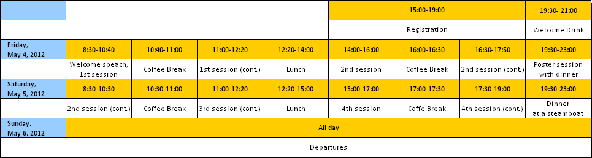
Program Overwiew